Direct Oxidative Stress Damage Shortens Telomeres

5/14/2019
PITTSBURGH – The same sources thought to inflict oxidative stress on cells—pollution, diesel exhaust, smoking and obesity—also are associated with shorter telomeres, the protective tips on the ends of the chromosomal shoelace.
 A new study from the University of Pittsburgh, published today in Molecular Cell, provides the first smoking gun evidence that oxidative stress acts directly on telomeres to hasten cellular aging.
A new study from the University of Pittsburgh, published today in Molecular Cell, provides the first smoking gun evidence that oxidative stress acts directly on telomeres to hasten cellular aging.
“Telomeres consist of hundreds of guanine bases, which are sinks for oxidation,” said senior author Patricia Opresko, Ph.D., professor of environmental and occupational health at the Pitt Graduate School of Public Health and UPMC Hillman Cancer Center. “Is it just a coincidence? Or could it be true that oxidizing those guanines in the telomeres is really contributing to shortening?”
To find out for sure, Opresko needed some way to inflict oxidative stress on telomeres and nowhere else.
So, she enlisted the help of Marcel Bruchez, Ph.D., professor of biological sciences and chemistry and director of the Molecular Biosensors and Imaging Center at Carnegie Mellon University. Bruchez developed a method for zeroing in on the telomeres using a special light-activated molecule that latches onto the telomere and delivers localized free radicals—the molecular agent of oxidative stress—on command.
“One of the main challenges to targeting oxidative damage to specific loci in living cells has been achieving precise temporal and dose-control of this damage,” Bruchez said. “By combining telomere targeting with our optochemogenetic generation of singlet oxygen, we are able to selectively control when and how hard the oxidative stress is applied specifically at the telomere sites.”
 The researchers repeatedly exposed cultured cancer cells to this targeted oxidation procedure, mimicking conditions of environmental oxidative stress and inflammation, and, indeed, they saw the telomeres break and shorten with each cell division, despite repair efforts by the telomere lengthening enzyme telomerase.
The researchers repeatedly exposed cultured cancer cells to this targeted oxidation procedure, mimicking conditions of environmental oxidative stress and inflammation, and, indeed, they saw the telomeres break and shorten with each cell division, despite repair efforts by the telomere lengthening enzyme telomerase.
As the DNA repair machinery tried to fix the broken telomeres, the ends of the chromosomes often fused together, destabilizing the genome and preventing cells from dividing properly.
Whereas telomere shortening spells bad news for healthy cells, Opresko said, the flipside is that targeting telomeres might offer a way to fight cancer. With short enough telomeres, cancer cells would stop dividing.
“If we can understand what causes telomere shortening and how cells compensate for that,” Opresko said, “then we’ll be in a better position to design intervention strategies that protect telomeres in healthy cells and target telomeres in cancer cells.”
Other authors on this study include Elise Fouquerel, Ph.D., Ryan Barnes, Ph.D., Shikhar Uttam, Ph.D., and Simon Watkins, Ph.D., all of Pitt.
This work was supported by grants from the National Institutes of Health (K99ES027028, R01ES022944, R01CA207342, R01ES02842, R21/R33ES025606 and R01EB017268).
VIDEO INFO:
Title: Telomere damage causes failed cell division, death.
Caption: A 24-hour time lapse movie following light-induced oxidative telomere damage shows how daughter cells get stuck together by DNA fusion and ultimately die.
Credit: Fouquerel et al. (2019). Mol Cell.
PHOTO INFO: (click image for larger version)
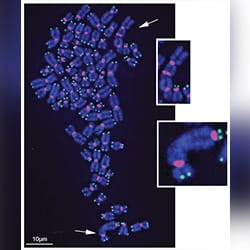
Title: Damaged telomeres get stuck together.
Caption: Chronic oxidative damage to telomeres (green) on the ends of chromosomes (blue) causes chromosome fusions (white arrows).
Credit: Fouquerel et al. (2019). Mol Cell.
For Journalists
Cynthia Patton
Director
412-415-6085
pattonc4@upmc.edu

